Impurity management continues to be a major challenge in drug development—compromising purification, reducing yields, and raising safety concerns. These hurdles often lead to higher costs, longer timelines, and increased regulatory risk.
At SpiroChem, we specialize in solving these challenges. Our experts use advanced analytical tools and synthetic chemistry to identify, characterize, and synthesize process impurities—ensuring your process is robust, scalable, and regulatory-ready.
Our typical impurity elucidation workflow is typically as follows :
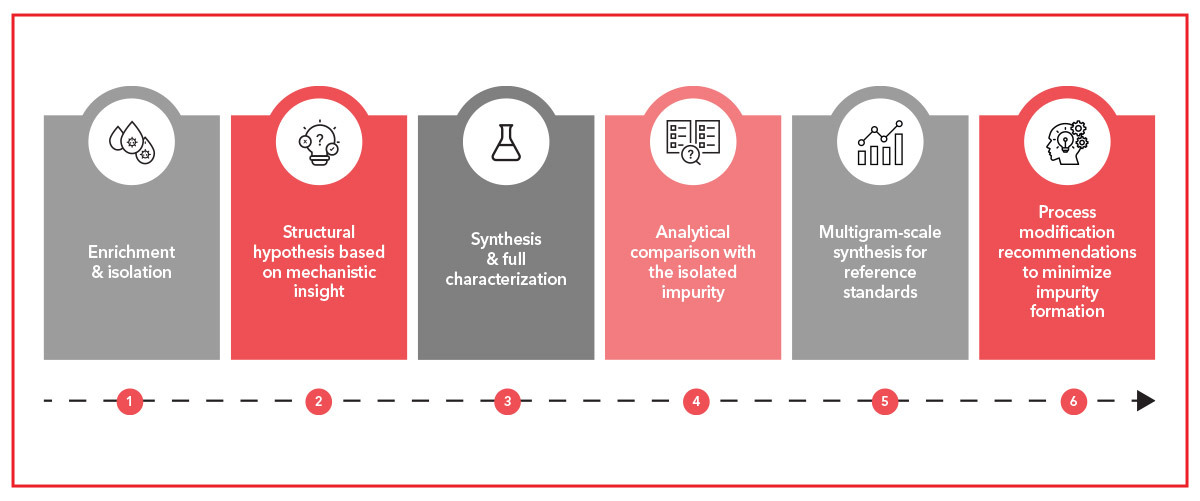
- Enriching and separating the impurity (recrystallization, flash chromatography, preparative TLC / HPLC…).
- Proposing impurity structure based on process data and mechanistic insight.
- Synthetizing the likely impurity and providing full characterization.
- Matching the synthesized impurity with the isolated sample to confirm the structure.
- If confirmed, re-synthesizing the impurity at larger scale to be used as reference standard.
- Recommending process modifications to prevent or reduce impurity formation
Whether you're facing an unexpected impurity or building a control strategy from scratch, we’re here to help.






















